Chapter outline
- 4 main tissue types (by adult apperance)
- 3 main tissue types (by embryonic lineage)
- ectoderm
- mesoderm
- endoderm
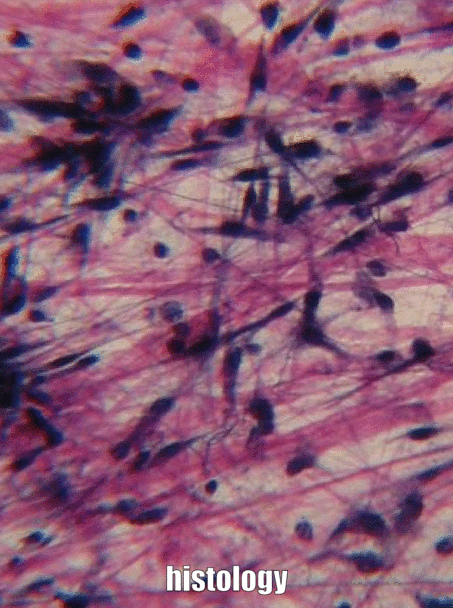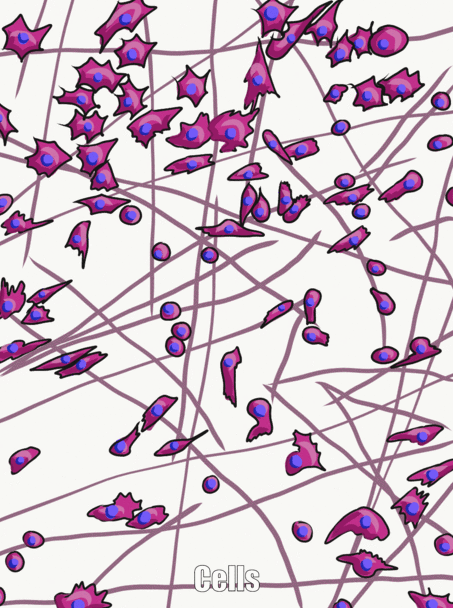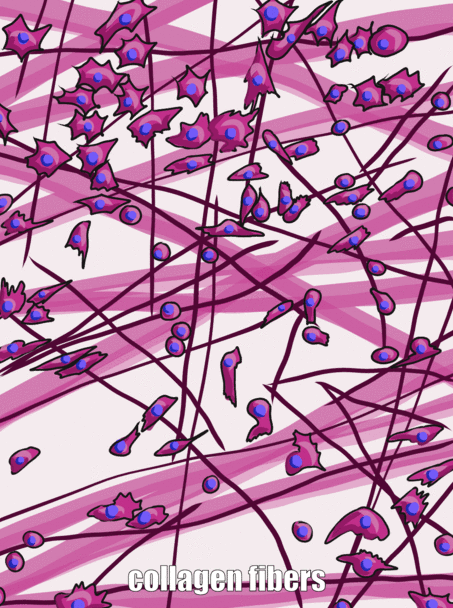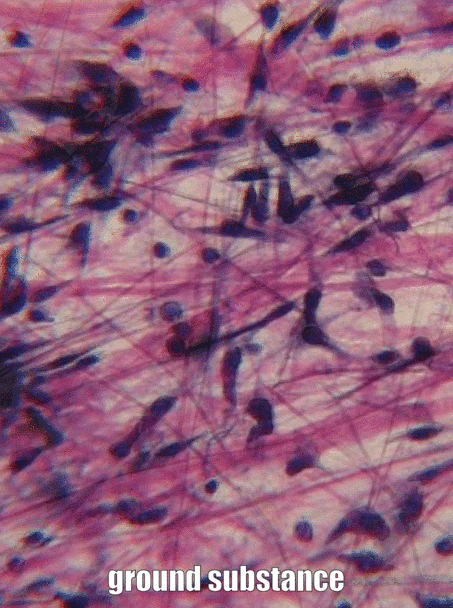
The aim of this chapter is to review histology covered in your Anatomy & Physiology classes-- but only the tissues that appear in the head and neck, and only the parts that are relvant to this course. This is by no means a comprehensive review. Histology is the study of what tissues look like under a microscope. A tissue is a group of cells, all of the same type, working together to perform a function. Because most cells are transparent, microscopists use of a number of different stains to highlight different parts of cells. Shown to the left is an image of a very common staining technique called an H&E stain. These two stains turn molecules with negative charges blue, and positive charges pink. DNA and RNA take up both colors, whick makes nucleuses purple, while most proteins turn pink. Unfortunately, most cells are full of proteins so their cytoplasm turns pink, but the extra-cellular matrix is also full of protein, it turns pink as well. We are therefore often trying to make sense of a sea of pinks with purple spots. On the plus side, if you purchase a histology coloring book, you only need two crayons.
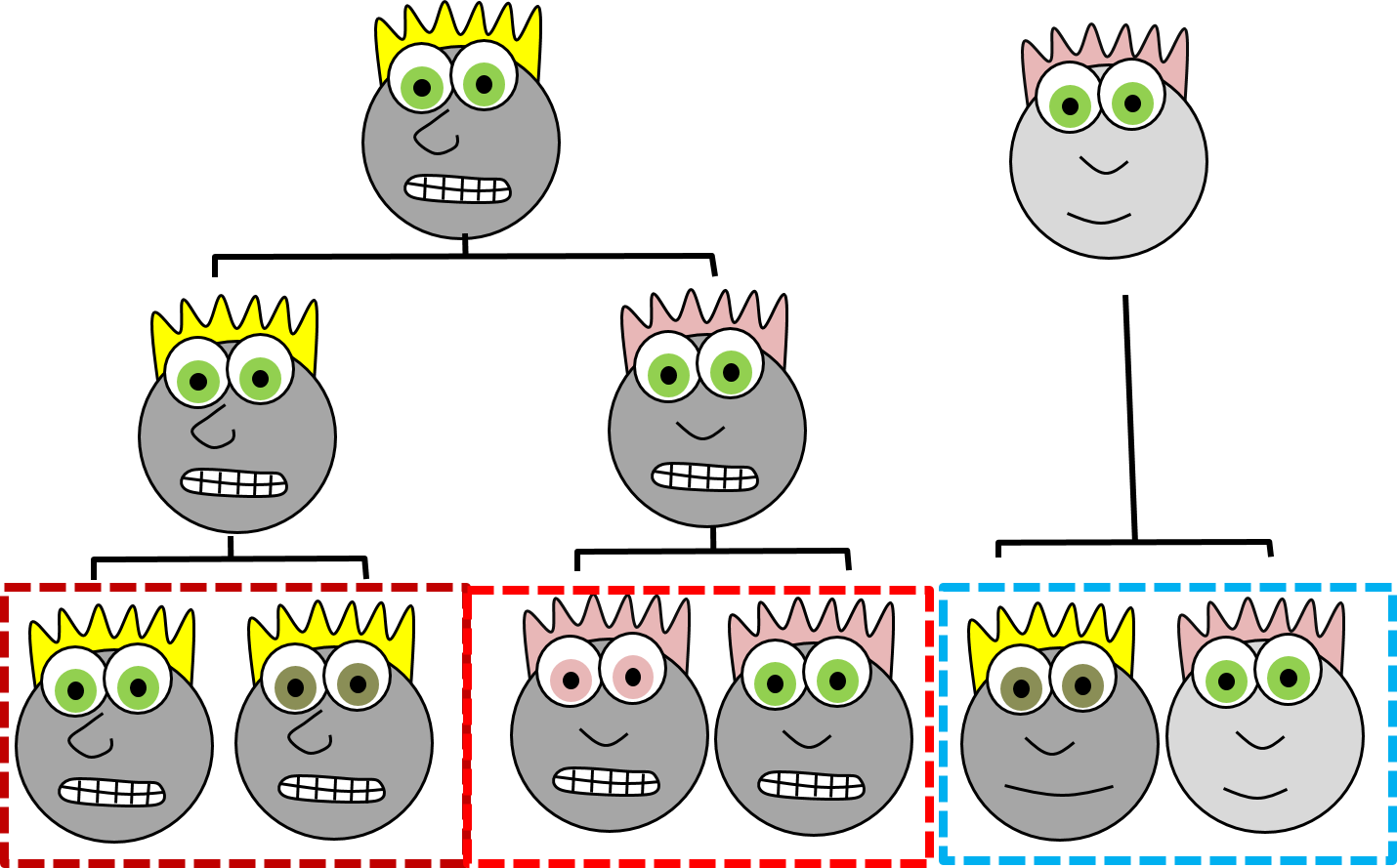
Rather than use the appearance of our cartoon people, if we knew their lineage (you might also see the fancier term ontogeny), we can classify them into two more accurate families, with one sub-family. When I use the word lineage, think family line, where differentiated cells are the youngest generation, and stem cells are their parents and grandparents. But, sadly, this is not how histology is taught, so we're going to look at what adult cells look like and classift them that way. When we do, look to see whether two tissues have distinct borders (different lineages) or blended borders (often the same lineage).
| Epithelial |
| Connective Tissue |
| Muscle |
| Nervous |
s
| Number of layers | Shape of cells |
| simple | squamous |
| stratified | cuboidal |
| columnar |
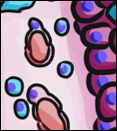
If you look at the lining of a capillary, you might be able to see the simple squamous epithelium that lines the inner surface. These cells, called endothelial cells, are functionally really cool cells, even if they are hard to see. Normally, endothelial cells keep blood inside of a capillary, but they can become damaged, such as during probing or flossing.
Simple cuboidal epithelia line most exocrine gland ducts, such as sweat and salivary glands. In the image to the left, you can see two ducts coming out towards the camera, and one duct running side-to-side. Each is surrounded by a simple cuboidal epithelium. Where two ducts get close together, it is still simple cuboidal epithelium, just two seperate layers of it. The capillary we saw two sections above is lined by flat cells to allow for diffusion of nutrients out of the capillary, but these salivary ducts have thicker cells to keep saliva in the duct.
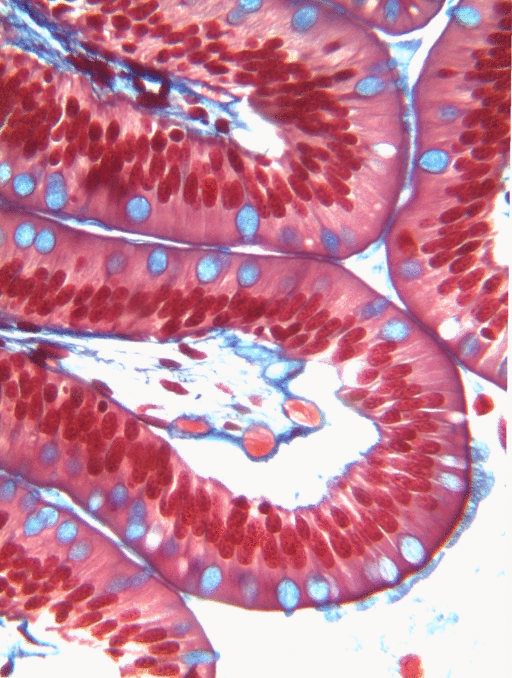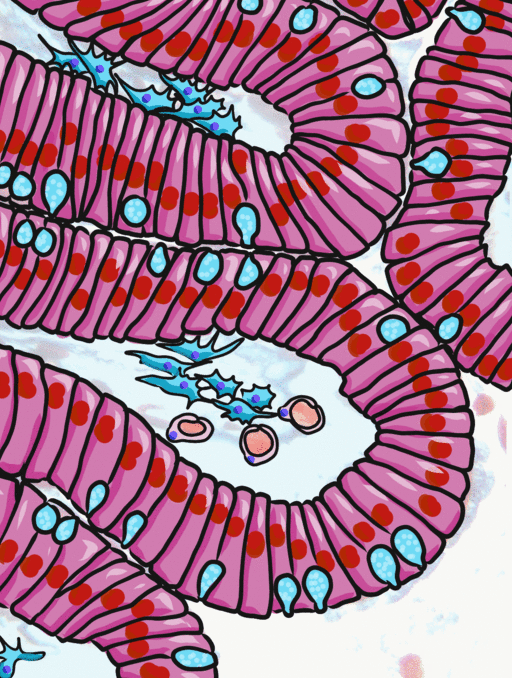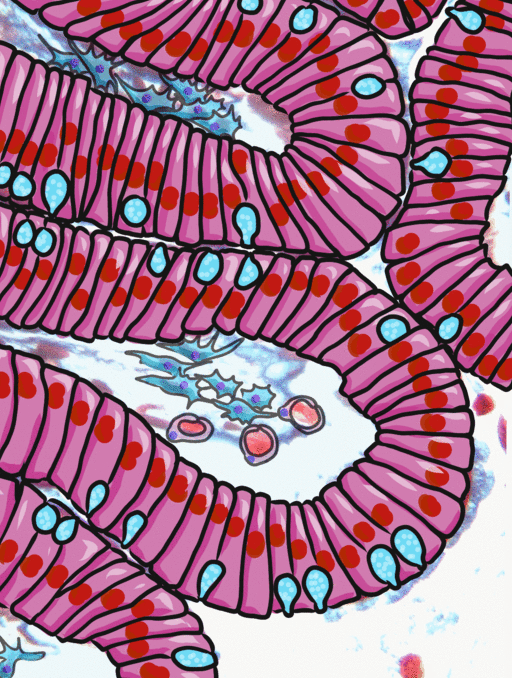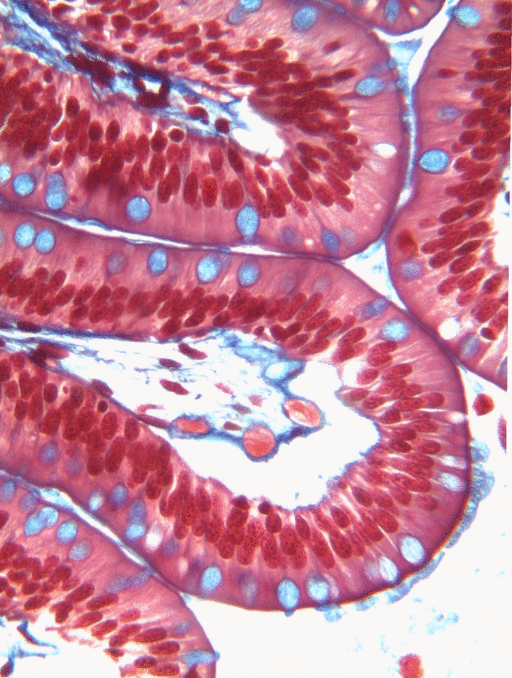
Simple columnar epithelia will not be found in the oral cavity of your patients, but we will see some this term when we focus on the Inner Enamel Epithelium that differentiates into enamel-producing ameloblasts. When we do, the cell shape won't be important, other than it allows scientists to find the IEE in a developing tooth.
If you trust my skills in histology, feel free to skip this entire paragraph. But if you are looking at my illustration to the left, you may wonder why I drew fewer nucleusses than are visible in the actual histology picture. Did I mistakenly give you a picture of a stratified columnar epithelium and try to pass it off as simple? No. When we say an epithelium is simple, it means one cell thick from apical-to-basolateral. But when we cut a tissue sample to view under the microscope, our slice may be several cells thick from side-to-side. And when we lay the tissue sample on its side, the cells that were running side-to-side are now sitting on top of one another. I have illustrated what it should look like if we had the ideal 1-cell-thick slice of tissue. This might be a good time to bring up perspective. Epithelia form flat sheets, and here we are looking at a (folded) sheet in cross section. Imagine slicing through your bed and looking at it from the side view. You could make out the individual, single layers of your bed sheet and comforter. Furthermore you might note that while each is a single layer, the bedsheet is thin and the comforter is thicker. Under the miroscope, we're doing the same thing, but unfortunately cells are semi-transparent, so it gets messy. When we call this a simple epithelium, that means there is only one pink cell between the white space (here, the lumen of the intestines) and the very light-blue space (the connective tissue, where nutrients can be absorbed into blood vessels). Those cells, like the comforter, are thicker than cells we looked at above. But we can also see some columnar cells that were connected side-to-side in this cimple epithelium stacked on top of one another. Next time, you should probably just trust me.

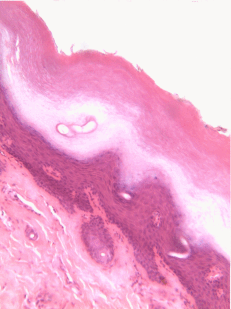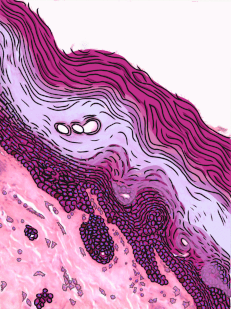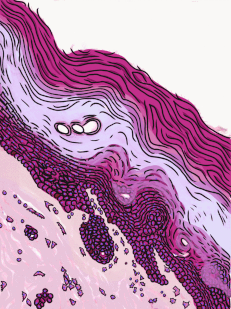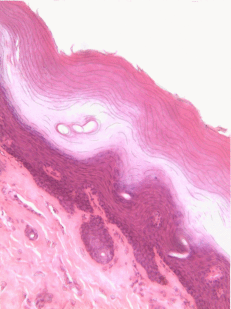
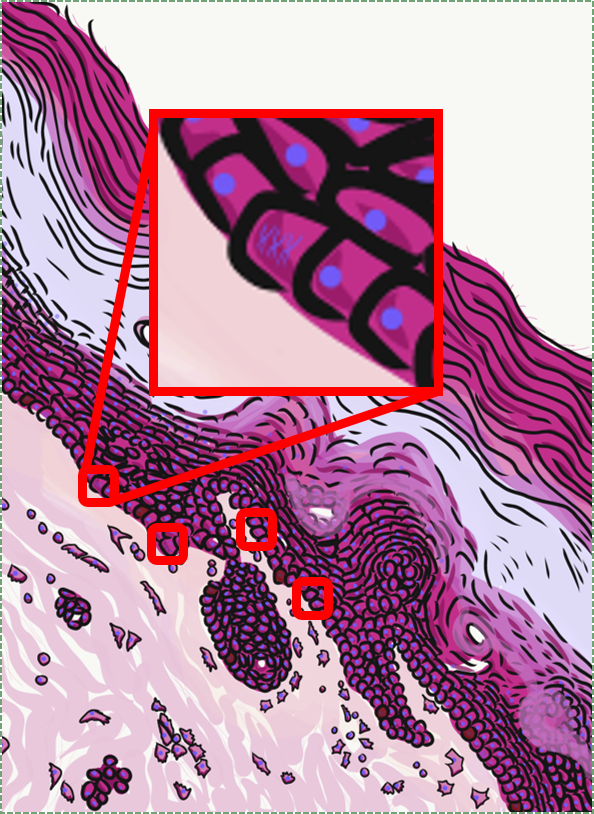
Stratified squamous epithelia can be found in the outer lining of the skin, and the inner lining of the oral mucosa. While we have different names for the skin and the oral mucosa, if we were to name them based off of embryology, rather than superficial appearance, they would both contain a stratified squamous epithelium. The epidermis is a keratinized stratified squamous epithelium. Keratin is a tough, water-resistant protein made by the main type of epithelial cell in this tissue, the keratinocyte. The stratified squamous epithelium changes at the vermilion border to less-keratinized, and the stratified squamous epithelium of the oral mucosa is either less or not-at-all keratinized. Based on that information, should I classify the vermilion zone as an extension of the labial mucosa, or as part of the skin and lips? Or is there a line in the vermilion zone that divides them into a skin half and an oral mucosa half? It's times like this I could use the advice of an embryologist to inform me I shouldn't be looking for magical lines in adult tissues, nor draw some sort of imaginary border between the skin, the vermilion and the oal mucosa based on color. If you ever change careers an go into proctology, you can use this same information down there.
You might spot some stratified cuboidal epithelium in a duct of larger exocrine glands, but I can easily describe these tissues: not appearning in this textbook.
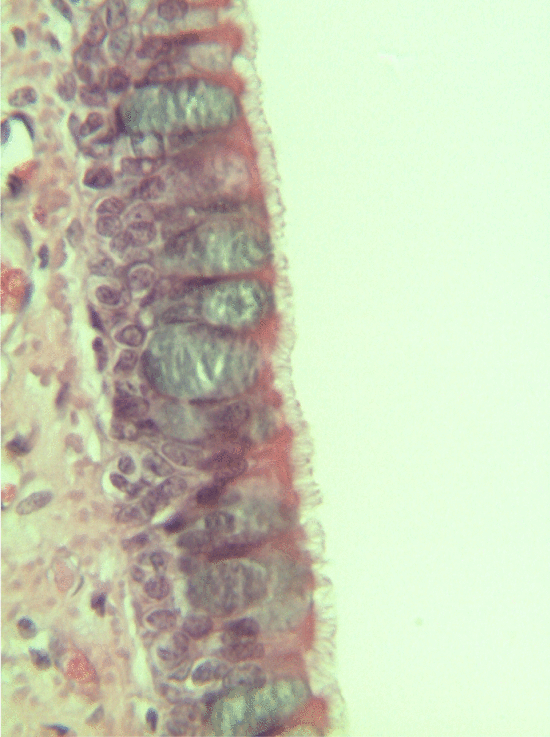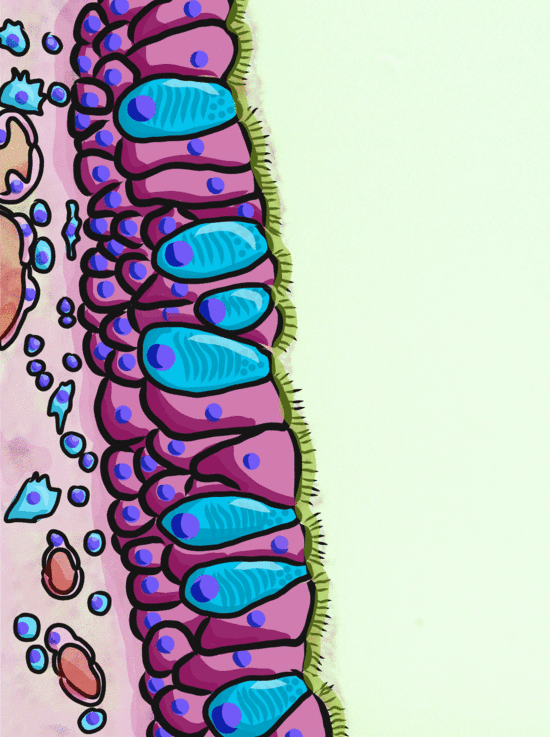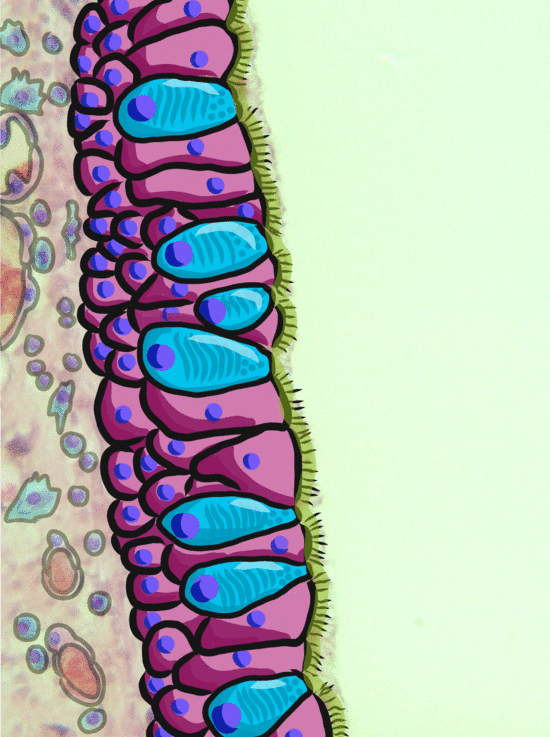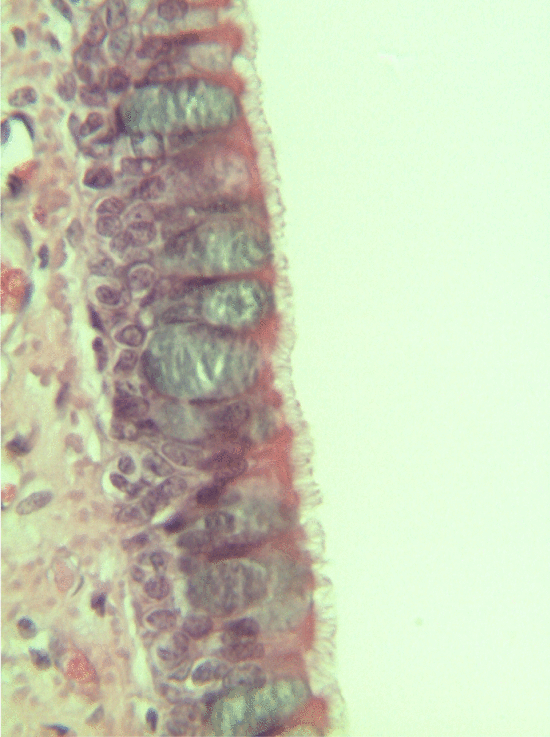
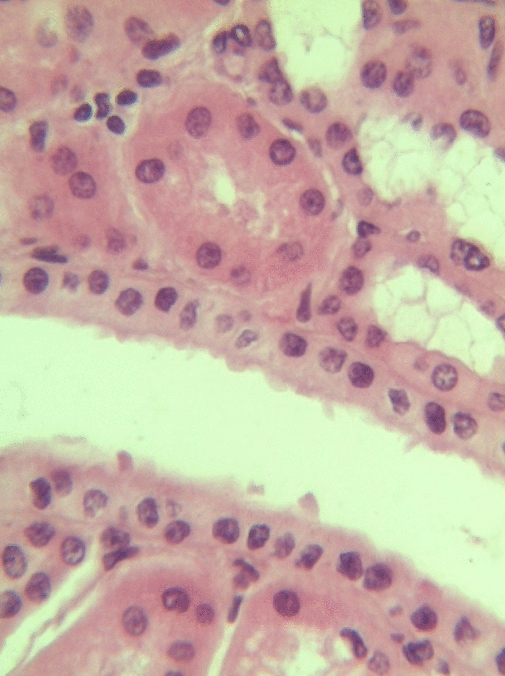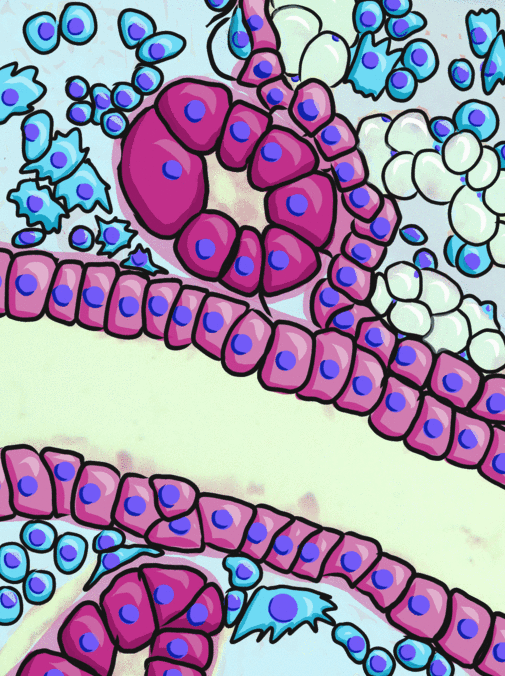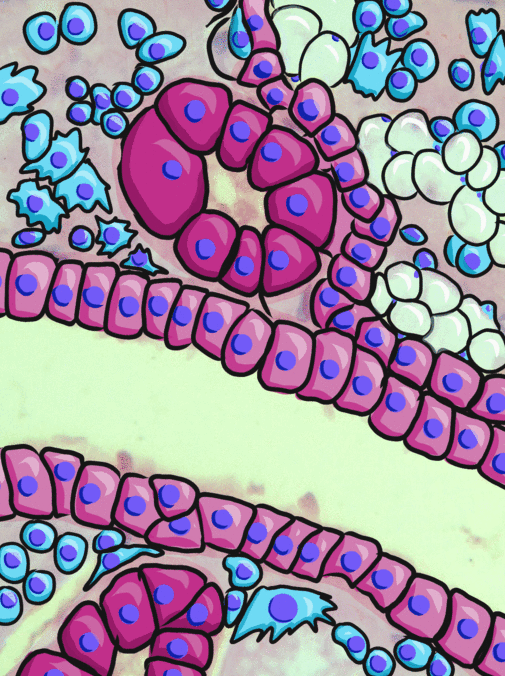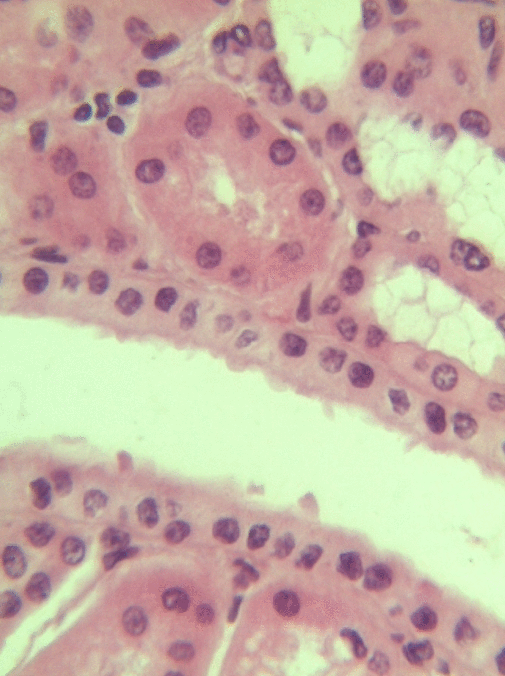
Ciliated pseudo-stratified columnar epithelium, or as most people say, pseudo-stratified epithelium, can be found in upper portions of the respiratory tract, such as lining the nasal cavity and the para-nasal sinuses. This tissue definitely deserves a different name from the stratified squamous epithelia of the skin, labia and oral mucosa. However, I'm not convinced it needed to break the naming rules I outlined above. It has more than one layer, but it is hard to count how many. So someone called this tissue pseudo-stratified.
The big fat blue cells, who don't have cilia, are named goblet cells. These cells produce mucus. Goblet cells synthesize mucous proteins within their rER and secrete them, where they attract water and become mucus. The blue coloring of the goblet cells tells us that mucous proteins do not carry the same ionic charges as most proteins found within the epithelial cells of the pseudo-stratified epithelium. Due to this difference in color, they are classified as a distinct cell-- a unicellular gland found within the pseudo-stratified epithelium. But what does our friend the embryologist say? Well, the columnar cells can't become goblet cells, and the goblet cells can't become columnar cells, but they do share a common ancestor (or stem cell), so we probably should consider the goblet cells a part of the pseudo-stratified epithelium. Does it matter? No, but questions like these will matter later in the book, it's good to start practicing now.
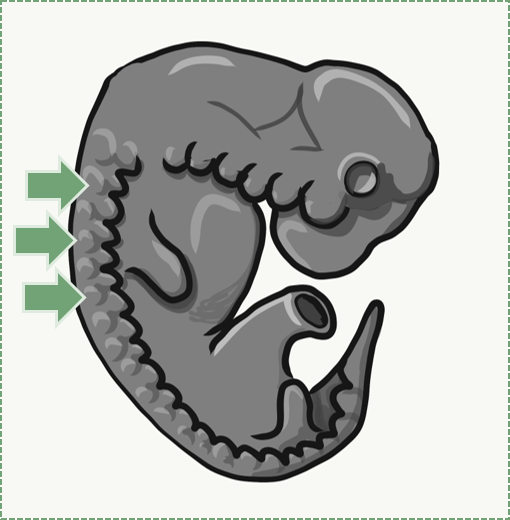
Many textbooks will tell you connective tissue connects 2 other tissues . That's like saying your smart-phone connects you to other people. It is technically true, but glosses over the fact these could be people you know, people you don't know, or people you don't want to know. It ignores the fact you could be communicating in real-time with people on the other side of the world, or accessing information from the near sum-of-all human knowledge (the internet), or reading the sophmoric rant of some disgruntled knuckle-dragger who, years ago, posted a rude comment on a youtube video recording of a histology class lecture (also the internet. Congratulations, Steve, you made it into my book... REVENGE IS MINE). So far I've tried to keep you, the reader, safe from extraneous information about histology that will never be relevent to you. Here I must go into more detail than your average undergraduate histology class. Do you trust me?
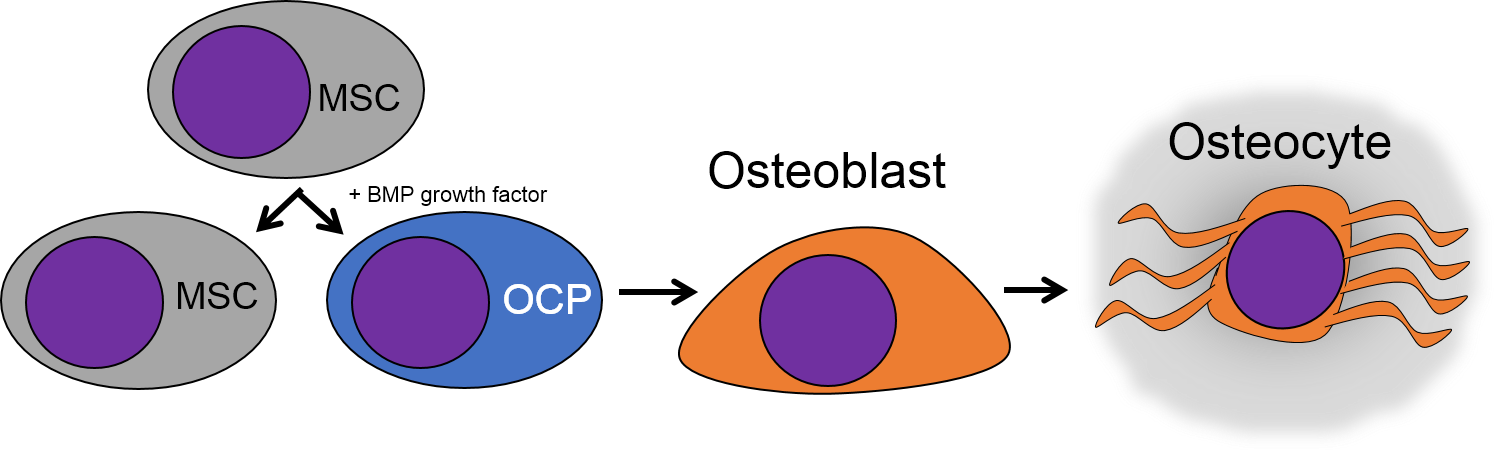
There are a number of different cell types found within a connective tissue. The stem cells are called Mesenchymal Stem Cells (MSCs). MSCs are extremely important cells (here is some further reading), and since you can find them in adult tissues they are also called a type of adult stem cell. These cells are capable of undergoing mitosis to produce more adult stem cells, which can differentiate into fibroblasts, lipoblasts, chondroblasts, osteoblasts, hemocytoblasts, myoblasts and neuroblasts. Thus they can form most connective tissues, including bone, cartilage, blood (both red and white blood cells), plus muscle and neural tissue. That's pretty much everything except epithelia, which MSCs can form as well after going through a transition.
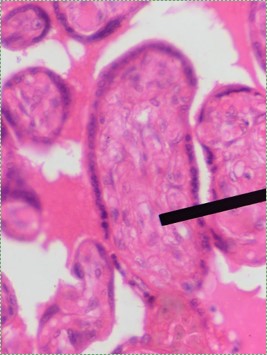
Mesenchyme is the first type of connective tissue we make. It turns into other connective tissues. It is an embryonic tissue that has yet to decide what it will become when it grows up. It is composed mainly of MSCs and some mucous ground substance. There is a special type of mesenchyme that we will run into, called ecto-mesenchyme. The lineage of ecto-mesenchyme is not from MSCs, but from a special type of neuronal cell called a Neural Crest cell. Neuro-mesenchyme forms other connective tissues I have yet to mention: dentin, cementum and the periodontal ligament.

Areolar CT is the quintessential connective tissue, or the most boring depending on how you look at it. It contains a little of everything the other connective tissues have: cells, ground substance, and all 3 fibers. Because it has a fair amount of ground substance, it is an ideal tissue to occupy places where blood vessels might need space to grow in the future. Hence areolar CT is found in regions that are highly vascular. This is why you can find a small layer of areolar connective tissue directly underneath nearly every epithelium, including the stratified squamous epithelium of the various types of oral mucosa.
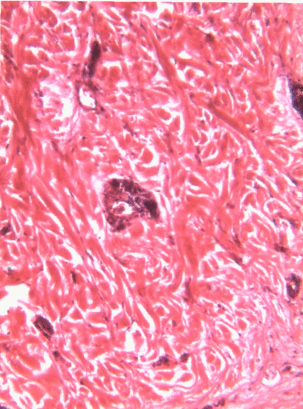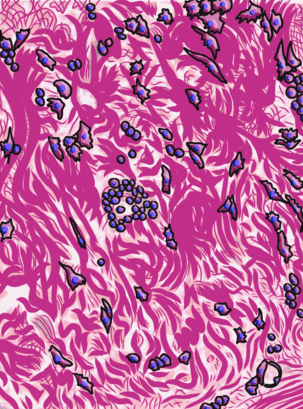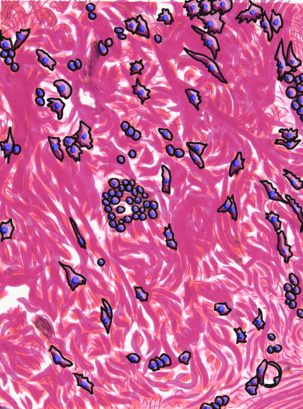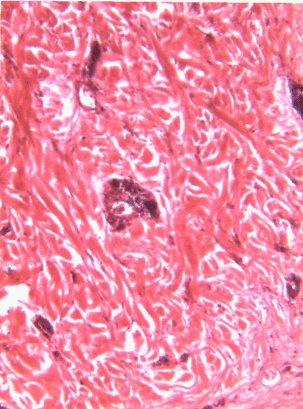
Underneath the areolar CT of the oral mucosa is dense irregular connective tissue. It contains fibroblasts which make the bulk of the tissue: collagen fibers, the strongest of the 3 fiber types. In a dense irregular CT, the collagen fibers point in all directions. This makes this tissue particularly strong in all directions. You can also find dense irregular CT (along with its buddy, areolar CT) in the dermis of the skin. Why in both places, you ask? lineage.
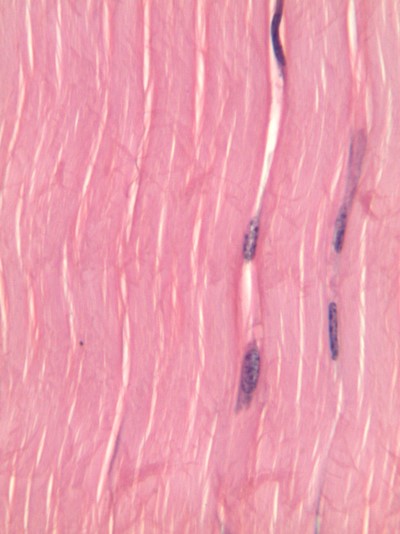
Like dense irregular CT, dense regular CT is mostly collagen fibers, only the fibers run parallel. That makes this tissue strong in one direction. You mostly find dense regular CT between muscle and bone, or between bone and bone. The only place in this book where we will find dense regular CT is the periodontal ligament, between bone and cementum.
If you trust my histology skills by now, you can skip this paragraph. The rest of you might be thinking the collagen fibers look wavy, not parallel. Well, when someone sliced this section of a tendon, they had to saw through the tissue using a razor blade. That blade pushed the fibers in one direction, then pulled them back, and pushed again as the person was was cutting as thin of a slice as they could. If they wanted better results, they should have used an expensive device such as a microtome, cryotome, vibratome or vibraphone (just kidding, that last one is a percussion instrument). I bring this up because many textbooks suggest histology is a nearly-perfect representation of what is found in the human body, when in reality there are often numerous artifacts, or errors, that histologists have learned to ignore when they see them. That's why they used to pay me the big bucks in academic research, it was my job to find the best pictures with the fewest artifacts, because most of my audience wouldn't ignore them. That's why I've chosen to illustrate most of the images used in this book. Do you trust me yet?
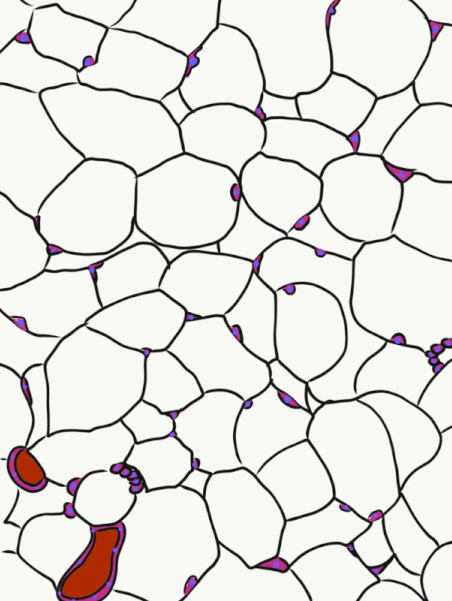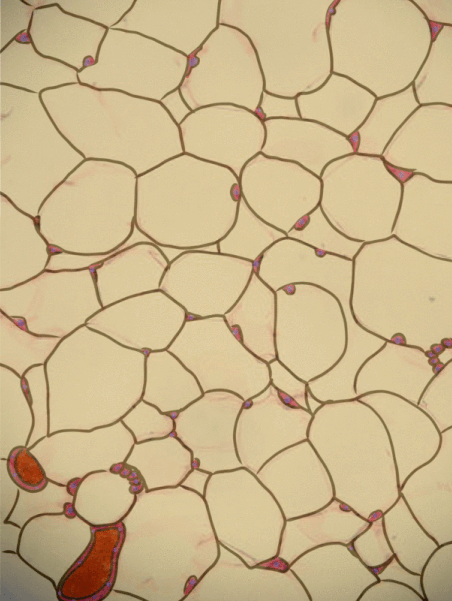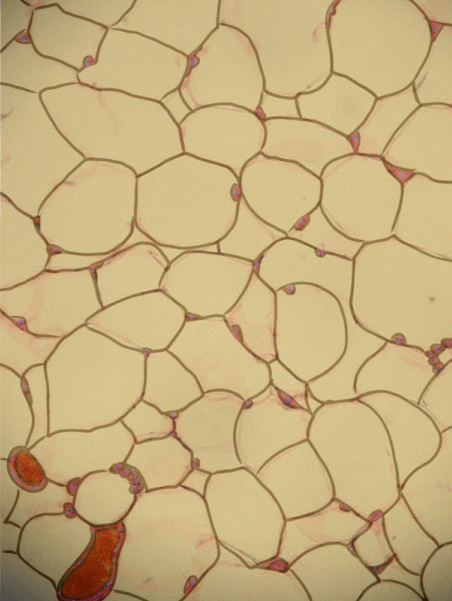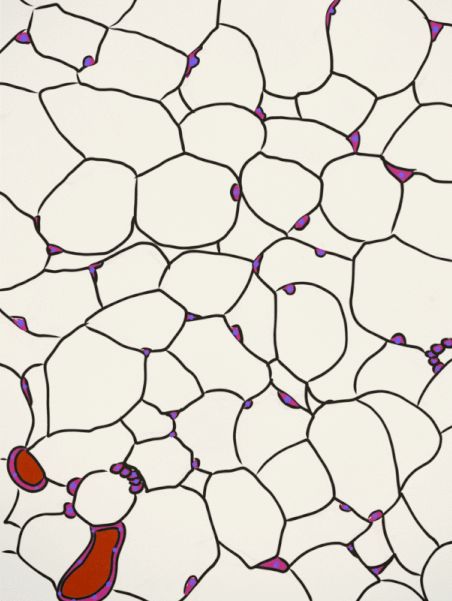
Adipose tissue is a type of connective tissue. It is special in that is has very little ECM. It contains mostly adipocytes, the mature cells that store triglycerides. It also contains MSCs, which if a person needs more adipose tissue, will divide and differentiate into adipocytes. Technically, the MSCs differentiate into a lipoblast first, but this would only be important for the sake of staying consistent with the names of other connective tissue cells. Maybe if they had named the cells adipoblast and adipocyte, we'd use the -blast name more frequently, but someone messed that up.
Most textbooks place adipose tissue next to areolar CT and categorize them both "loose", on account of their low number of visible fibers. That's fine if you like categories based on appearances, but you don't need to call either of them "loose". It is purely descriptive and does not correspond to anything physiological. We find adipose tissue in many places throughout the body, either between two other tissues, or embedded within another tissue (such as the adipose you find in bacon). On the other hand, areolar CT is found next to dense irregular CT, which is why I've deviated from the grouping found in most textbooks.
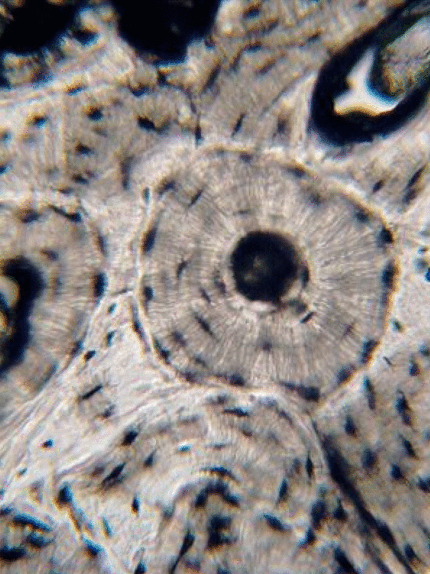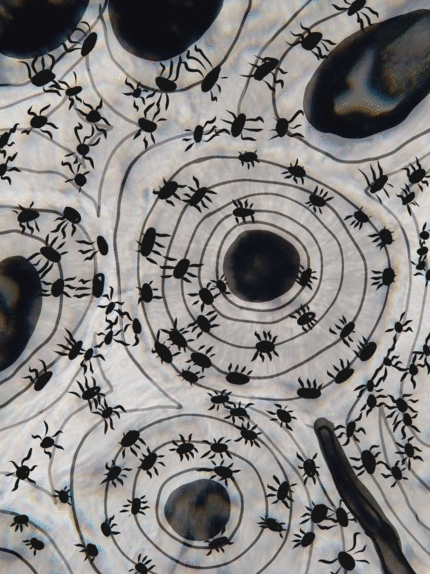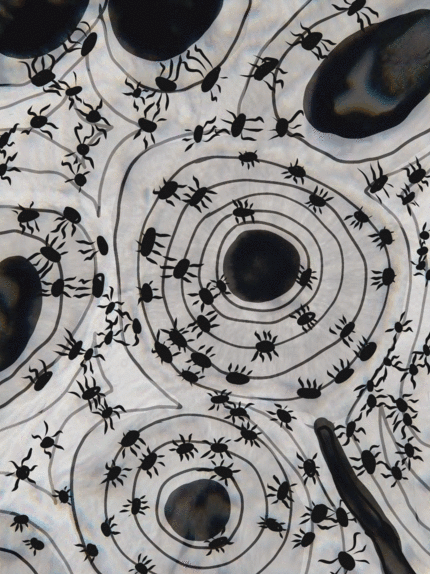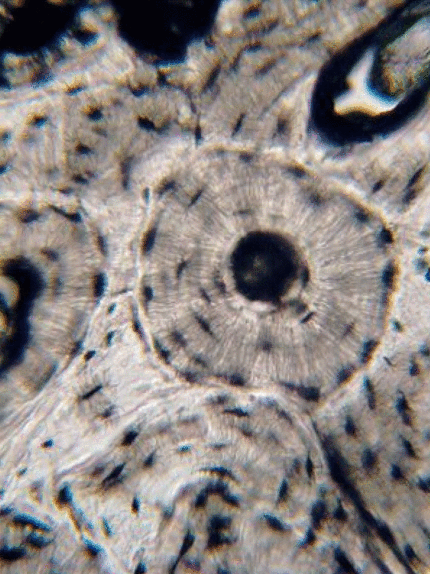
Bone (or osseus) is the last type of connective tissue I will cover in detail. In fact, because of its similarity to dentin, cementum and enamel, I will cover bone tissue in a fair amount of detail. Like any connective tissue, bone tissue comes starts as MSCs, which differentiate into an intermediate stem cell called an osteo-chondro-progenitor cell, which can in turn decide to become an osteoblast or a chondroblast, depending on what signals it receives. It can also remain a stem cell within the connective tissues surrounding bones, the periosteum (superficial) and endosteum (deep). Osteoblasts secrete collagen fibers and ground substance, which later mineralizes, trapping the osteoblasts inside of a lacuna. At this point, they differentiate into osteocytes and maintain the bone tissue. Next, a new set of osteoblasts lay down another layer of bone tissue around the previous one. This creates either the concentric layers of bone tissue found in the osteons of compact bone, or the thinner spiral layers of bone tissue found in the trabeculae of spongy bone.
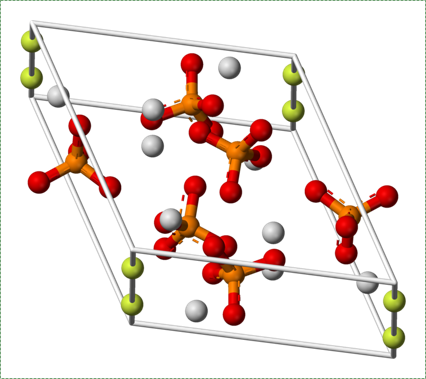
Collagen fibers run parallel within one layer of bone tissue, but run 90° in the next layer. While the ineral component of bone and tooth tissues provides strength, the collagen provides the ability to bend or flex, readucing the chances the tissue will shear off under stress. Collagen fibers, therefore, have a function similar to the rebar in reinforced concrete. The lower percentages of collagen found in enamel makes it harder and more resistant to caries than dentin or cementum, but more succeptible to abfraction.
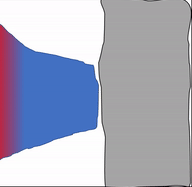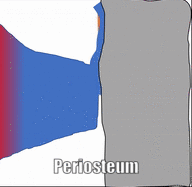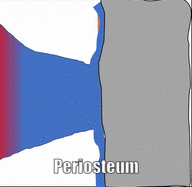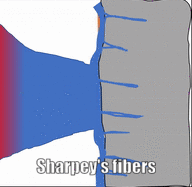
Osteoclast activity is very important during the exfoliation of teeth, as bone tissue is removed to loosen the connection between bone and tooth. Osteoclasts activity is also key to the mechianism by which orthodontia works. Lastly, osteoclasts play an important role in maintaining bone health-- this may seem counter-intuitive, because they destroy bone tissue. Bone tissue is constantly being repaired by a group of osteoblasts and osteoclasts working together, known as a remodelling unit. Because compact bone tissue is dense, there is little room for cells to work. Osteocytes can repair small amounts of damage, but larger amounts of damage would build up over time. To prevent this, remodelling units constantly work to remove bone tissue and replace it with fresh bone tissue. The remodelling units cannot find damaged bone tissue, they simply keep removing and replacing bone tissue. Physical stress on bone tissue causes osteoblasts to work a bit harder, which leads to increased bone density. Loss of osteoblast-stimulating hormones (such as estrogen) can make osteoblasts work a bit slower than the osteoclasts, which can lower bone density and potentially lead to osteoporosis.
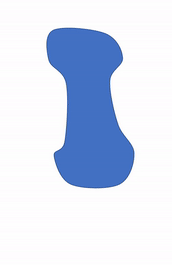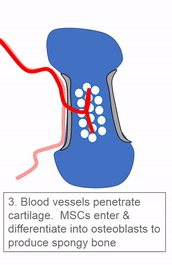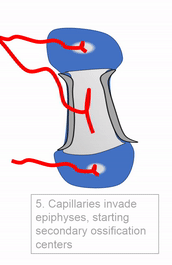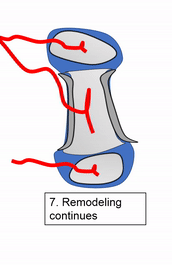
Most of the skeleton starts off not as bones, but as cartilage. Cartilage is typically avascular, but when chondrocytes receive the correct signals, blood vessels can grow into the ground substance. The growth of new blood vessels into a tissue is called angiogenesis. Aniogenesis allows MSCs to migrate into the central area and differentiate into osteoblasts and begin replacing cartilage tissue with bone tissue. This first site of ossification is named the primary ossification center. As remodelling occurs at the primary ossification center, blood vessels grow into the epiphyses and begin the same process at what are named the secondary ossification centers. Ultimately, most of the cartilage is removed, except in two important places. First, cartilage may remain between the parimary and secondary ossification centers, leaving growth plates (such as in bones of the arms and legs). Cartilage also remains at the very ends, to produce the articular cartilage found in a synovial joint, such as the tempero-mandibular joint. Intra-membranous ossification, because it begins with a dense connective tissue, leaves fontanels (soft-spots) between bones until ossification is complete, rather than cartilaginous connections.
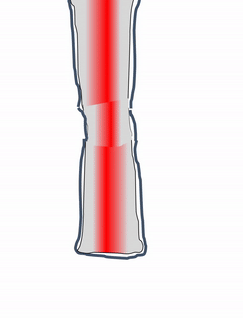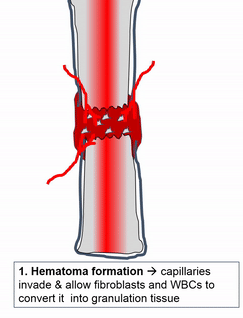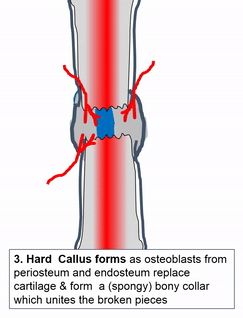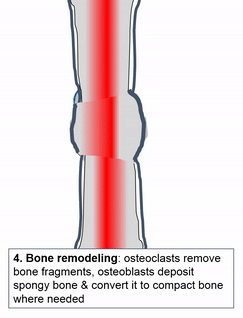
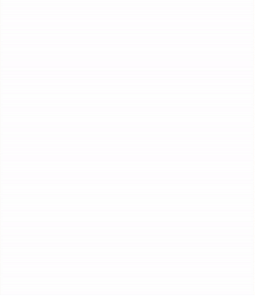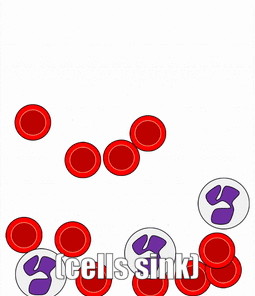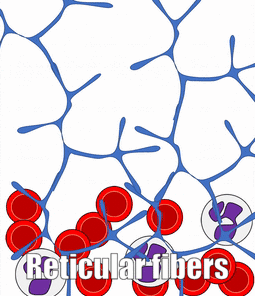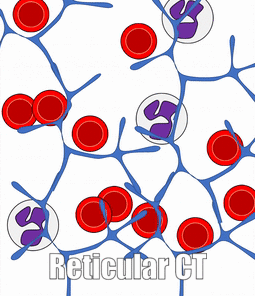
There are a number of other connective tissues covered in histology classes, I will cover them very briefly. Blood is a type of connective tissue. Reticular fibers are found in areas where blood cells tend to rest, such as in lymphatic organs, and together this is called reticular connective tissue.
There are some other forms of connective tissue that aren't typically covered in histology classes, and I would like to mention one of them: scar tissue. Scar tissue is made by fibroblasts and is very similar to dense regular CT, except the collagen fibers in scar tissue are highly cross-linked. This makes scar tissue very strong, but also reduces its mobility. Scar tissue can be formed wherever there are large injuries to an organ-- fibroblasts can often fill up the space more quickly than waiting for regeneration to occur. Once formed, scar tissue is very difficult to remodel, so it is more-or-less permanent.

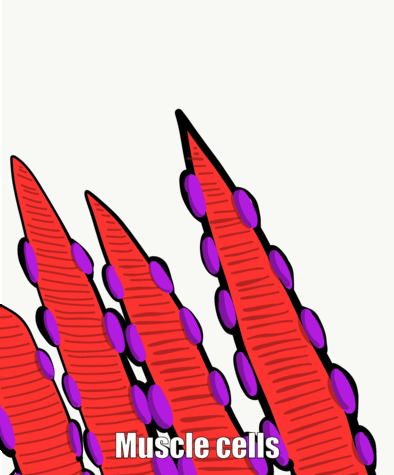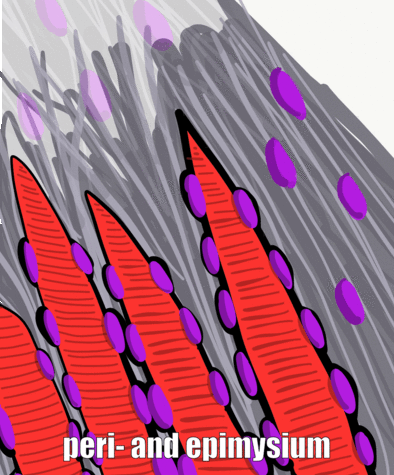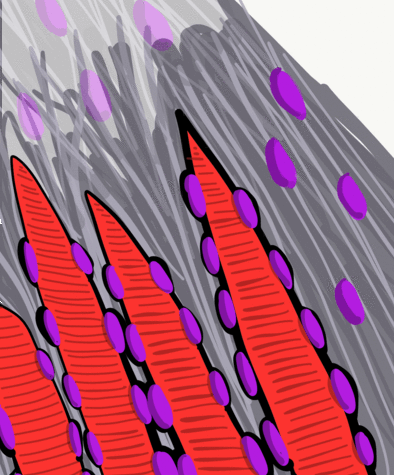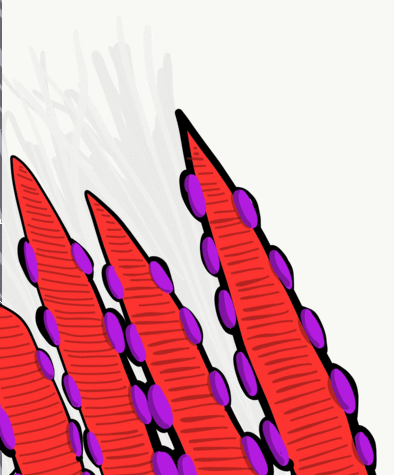
Muscle tissue, like connective tissue, is derived from somites. The stem cell that can differentiate into one of the several types of muscle cells is called a myosatellite cell. Given the correct set of extra-cellular signals, myosatellite cells differentiate into skeletal, smooth or cardiac muscle cells. Skeletal muscle cells are long multi-nucleate cells that were once hundreds of myoblasts that fuse together. They are often confusingly referred to as muscle fibers, but they are definitely cells, not molecules like the protein and glycoprotein fibers found within the ECM of connective tissue. In fact, they aren't the only multi-nucleate cell you read about in this chapter, so it perplexes me as to why this cell type needs a special name (whose name confuses me). In adult muscle tissue, some myosatellite cells remain, ready to undergo mitosis and fuse to form larger muscle cells in reponse to exercise. We will see skeletal muscle in the tongue and underneath regions of oral mucosa, but I will not be covering smooth or cardiac muscle tissue.
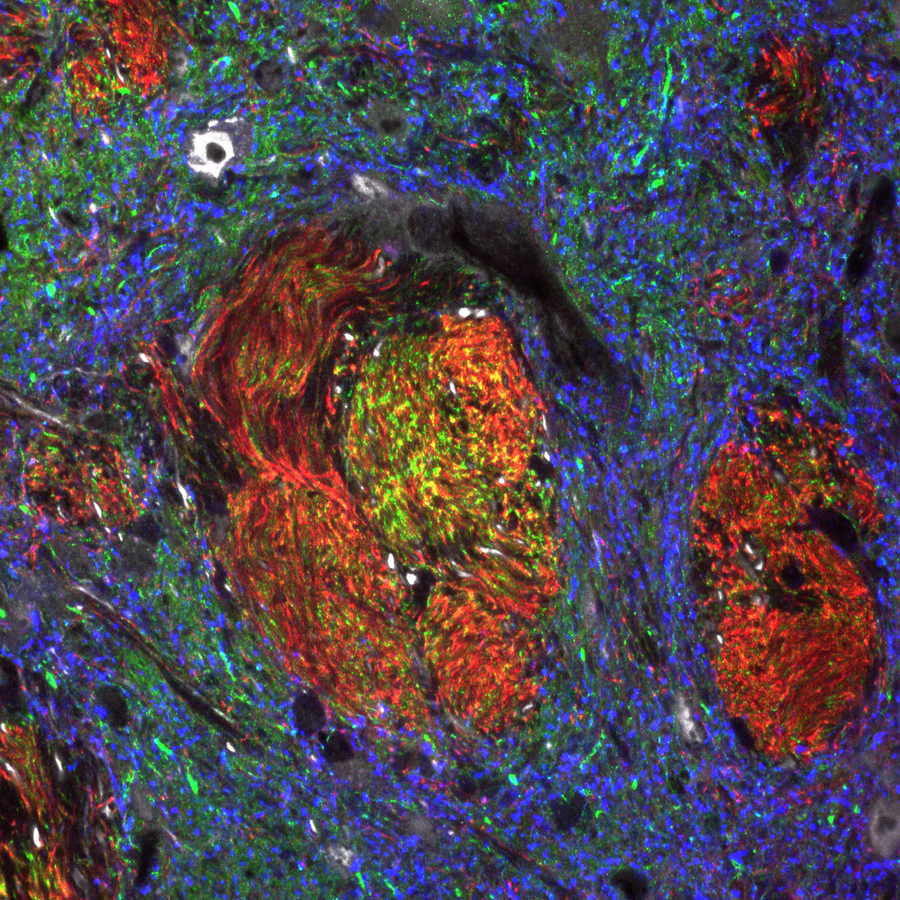
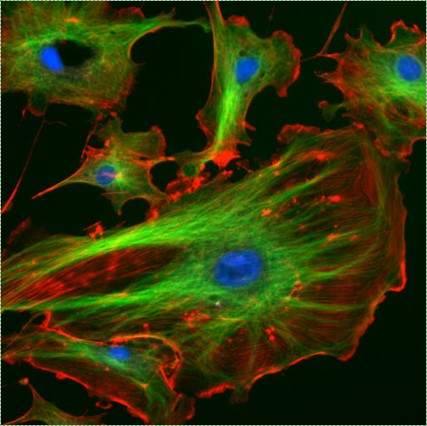
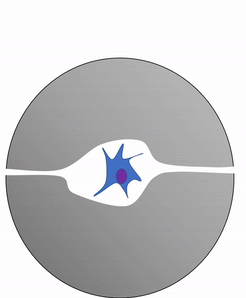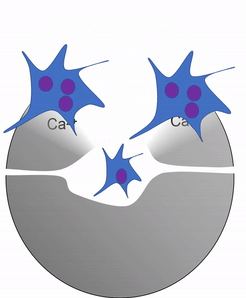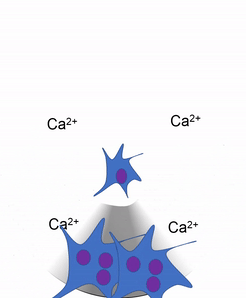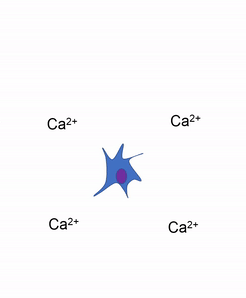
Shown to the left is a confocal image of neural tissue. What I like about this image, and is often absent from images of neural tissue, is that this one shows all of the cells. The typical image of neural tissue uses stains to highlight neurons, leaving out the glia. What you should see at this time is neural tissue is composed primarily of cells, and has very little ECM. While the brain especially has a huge number of blood vessels inside of it, technically they do not actually run within the neural tissue. Instead, there is a thin blood-brain-barrier separating the brain from blood vessels. Normally we wouldn't call neural tissue avascular, but I think I can here to make a point: neural tissue shares a lot in common with epithelial tissue. By now, I hope you have guessed why: neural and epithelial tissue share the same lineage.
When an embryo implants in the uterus, it is a hollow ball of identical, omnipotent stem cells that resemble a simple cuboidal epithelium. But soon, some of these epithelial cells migrate inwards, in a very important process called gastrulation. At this stage, we now have two distinct layers of cells, one on the outside and one on the inside, lining what will ultimately become the gut. Next, cells from the outer layer change and lose contacts with neighboring cells, and migrate into the middle. As this happens, they stop looking like epithelial cells and begin to look like mesenchymal cells. This produces three layers of cells in an embryo. The three layers are listed below, along with what they will form in the adult:
| 3 embryonic layers | Cell fate |
| Ectoderm | Epithelium of skin and oral mucosa, neural tissue |
| Mesoderm | Connective & muscle tissue |
| Endoderm | Epithelial lining of hollow organs |